The Pulsair Desktop Tonometer is a new product developed by ophthalmic product specialists, Keeler Ltd.
The Pulsair Desktop uses sophisticated optical and sensory technology for positional detection and puff-triggering authorisation. Its intelligent optical and electronic technology evaluates every reading to ensure the smallest range. When sufficient reliable readings have been taken, a time-saving audible signal alerts the user that the procedure is complete. The embedded software analyses the readings and eliminates any anomalies from the final display result.
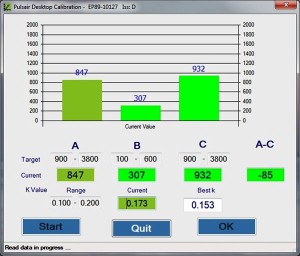
As it is such an intelligent device, a sophisticated calibration system is needed for factory calibration. Pulsair Desktop, like other medical devices, needs to be re-calibrated every year. However, if all of the Pulsair Desktop devices go back to the factory for annual re-calibration, it not only puts a huge strain on the factory calibration system but it also reduces the productivity of the devices and incurs a substantial cost from packaging and shipping.
A field calibration system is therefore required.
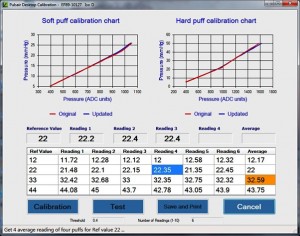
An “Electronics Eye”, which simulates the human eye’s optical properties under the Pulsair “air puff” pressure, has been developed at Keeler to calibrate the Pulsair Desktop in the field. This project has created a method to calibrate the Pulsair desktop using the “Electronics Eye” and developed dedicated software to be used for calibration of the equipment in the field.
The dedicated software must accommodate all of the requirements of the field calibration such as: giving the operator a step-by-step guide to the calibration ; providing assistance for optical system alignment; completing the calibration process automatically; inputting the calibration results table into the Pulsair Desktop EEPROM; generating, saving and printing the test report; fault diagnosis and recovery etc. All the requirements of the functions, the development procedure, documents, source code, verification and valuation, etc. must also be in accordance with FDA medical instrument of moderate level concern.